Bargain Sale Villaware Blender Purchasing Biphasic Defibrillator
Thursday, 29 September 2011
Philips / Laerdal HeartStart Onsite/Home/HS1 / / Project StatReview
Friday, 23 September 2011
AED-World.com Source of the world's largest EDA
Wednesday, 21 September 2011
Philips Medical Systems Heartstart FR2+ Battery - Model M3863A - Each
!±8±Philips Medical Systems Heartstart FR2+ Battery - Model M3863A - Each
Brand : Philips Medical SystemsRate :

Price :
Post Date : Sep 21, 2011 22:42:06
Usually ships in 1-2 business days
AED ForeRunner2 long-life LiMnO2 Battery
Saturday, 17 September 2011
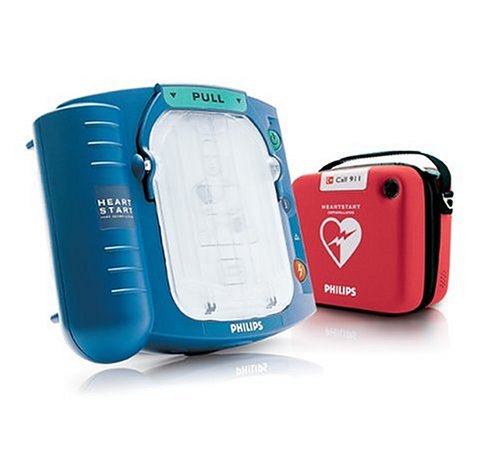
Philips HeartStart Home Defibrillator (AED)
!±8±Philips HeartStart Home Defibrillator (AED)
Brand : Philips Medical SystemsRate :

Price : $1,199.00
Post Date : Sep 17, 2011 15:34:33
Usually ships in 24 hours
Please Note: There will be a 0 re-stocking fee on all returns of this item.
Used to treat victims of sudden cardiac arrest (SCA), who are not responsive and not breathing normally. If in doubt apply the pads. Treats the most common cause of SCA by delivering a shock to the heart. Use HeartStart and CPR, as needed, until emergency professionals arrive.
Easy To Use - Guides you with interactive voice prompts
Safe - Delivers a shock only if needed
Reliable - Runs daily self-tests for readiness
Important: With sudden cardiac arrest, every minute counts. Make sure your HeartStart Home Defibrillator is set up and ready for use in an emergency.
Contents:
"Philips HeartStart Home Defibrillator (an automated external defibrillator or AED)"
Red carry case with 911/EMS card
Adult SMART Pads cartridge (lasts 2 years)**
Battery (lasts 4 years)***
Training video
5-year warranty
Facts you need to know about adult SCA:
For the best chance of survival, a defibrillator should be applied within 5 minutes.
Less than 5% of SCA victims survive. This is largely because defibrillators don't reach them in time.
Nearly 80% of all SCA's occur at home.
HeartStart user considerations:
You cannot use the HeartStart to treat yourself.
Users may need to perform CPR.
Responding to cardiac arrest may require you to kneel.
Voice instructions and enclosed materials are in English*.
HeartStart provides audible and visible indicators if the device requires maintenance.
If you have concerns about your health or an existing medical condition, talk to your doctor. A defibrillator is not a replacement for seeking medical advice.
Who should own a HeartStart?
Anyone who wants to be prepared.
Sudden cardiac arrest (SCA) is a leading cause of death in the U.S. The majority of victims have no previously recognized symptoms of heart disease - SCA typically strikes without warning.
A defibrillator will not
Promotional Samsung 2333hd Brand New Tissot Automatic Seastar Promotion Wenger Maxxum Backpack